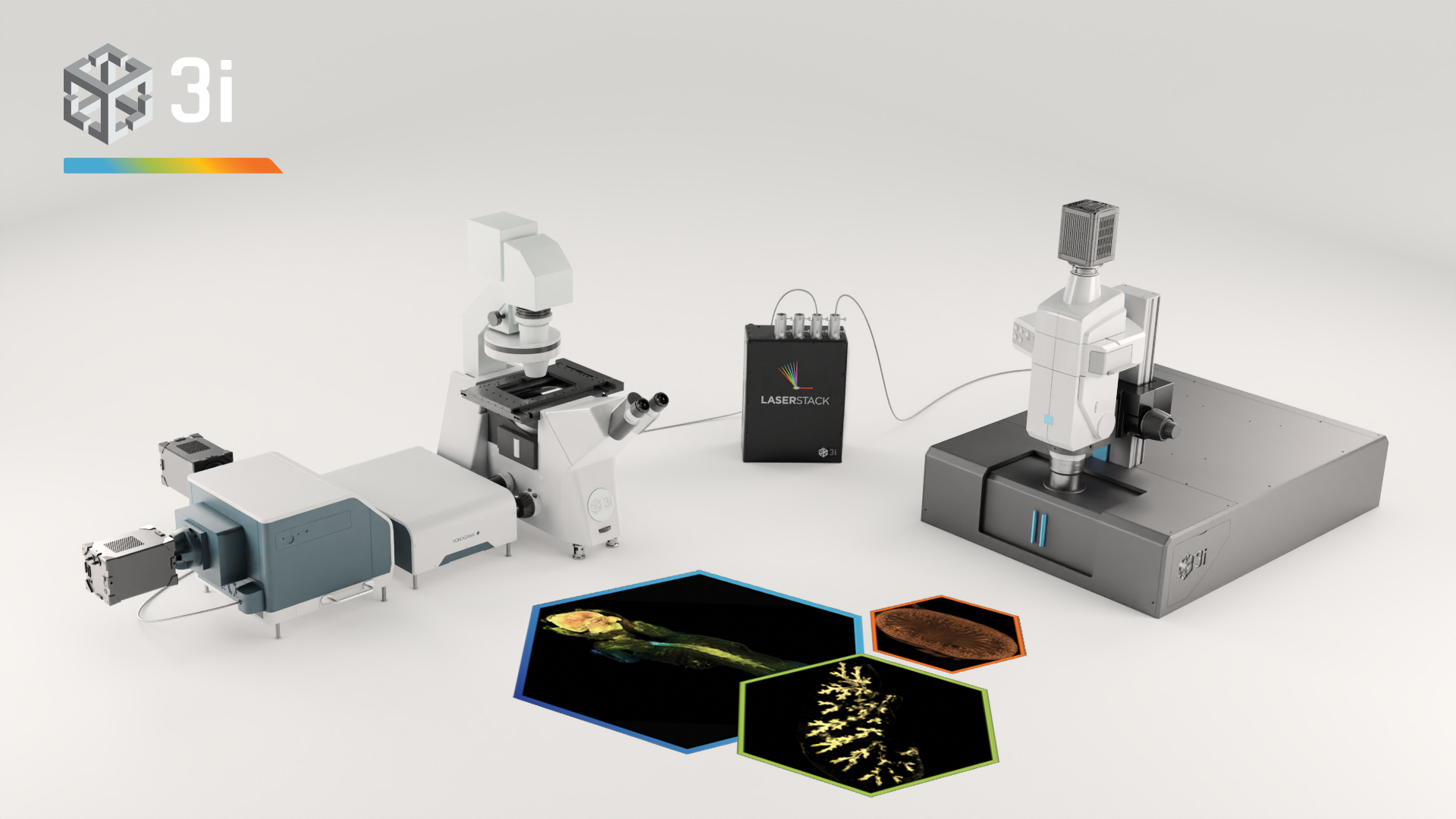
Clearing tissue is now a powerful and accessible technique for studying thicker samples in greater detail. The recent proliferation in tissue-clearing reagents and methods has made the technique more effective and more reliable over a greater range of samples from tissues to organs to whole animals. Once a suitable tissue-clearing regime has been established for a sample the following consideration is what image acquisition modality to use? Two potential options are a spinning disk confocal and a lightsheet microscope, both of which we will present in this workshop. Some of the parameters of a sample or the feature of interest will clearly direct this decision; for example, imaging a whole cleared mouse would typically preclude a spinning disk confocal and imaging a cleared tissue sample at super-resolution would typically preclude lightsheet microscopes. When imaging cleared specimens multiple factors need to be taken into consideration, foremost is a careful evaluation of the volumetric data such that it meets resolution requirements and is an accurate 3D representation of the sample. Other considerations are practical including: safety and ease of sample handling, ease of capture and duration of data acquisition. In this workshop we present our Marianas SDC inverted microscope system featuring a CSU-W SoRa super-resolution spinning disk confocal and our Cleared Tissue LightSheet (CTLS) microscope system and explore cleared tissue samples that could be imaged by each modality and compare the benefits and limitations of each technique.
Workshop 4
THU 9 JUNE 2022, time: 13:30 - 14:30
Click on the images to open the full image view!
Abbelight
Stand 36b
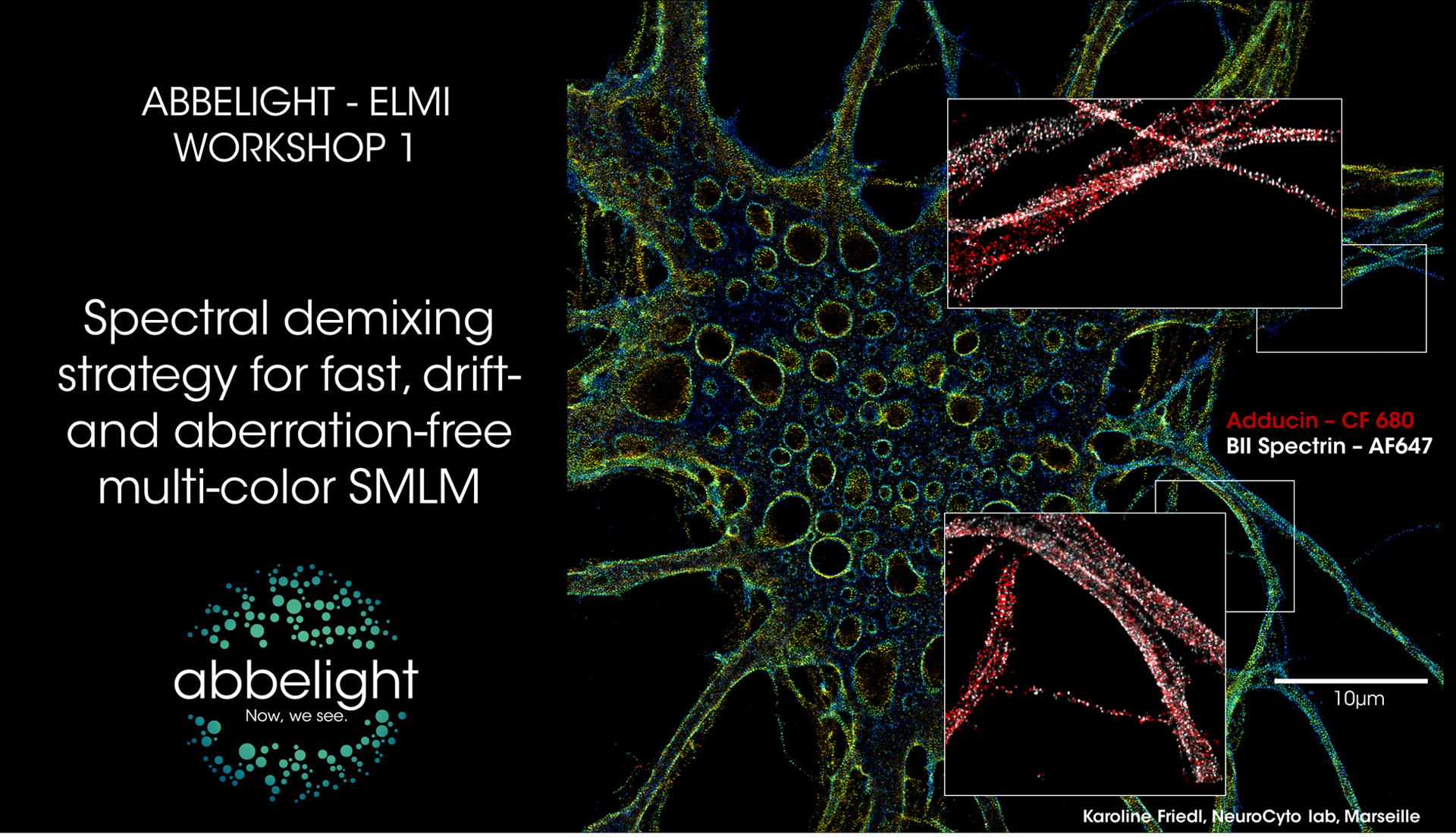
Spectral demixing strategy for fast, drift- and aberration-free multi-color SMLM
Different spectral demixing methods have been proposed by the SMLM research community: diffractive-based approaches offering a spectrum analysis of each single molecule in addition to its spatial location (Zhang, Z. et al. 2015) and ratiometric approaches (Bossi et al. 2008; Testa I. et al 2010; Baddeley et al. 2011; Lampe et al. 2012; Winterflood et al. 2015, Diekman et al. 2020) which are simpler and more robust. As simplicity and data robustness are two major focus of abbelight, the spectral demixing strategy adopted relies on the ratiometric method.
During this workshop, we will present the imaging by sprectral demixing with the SAFe 360 which the principle is to split the emission fluorescence into two distinct detection channels thanks to a long pass dichroic beamsplitter. By using fluorophores emitting within the same wavelength range, their partially overlapping emission is then spectrally separated by the dichroic and imaged onto two detection scientific-grade cameras (the choice of the dichroic is linked to the fluorophores and the SMLM strategy employed). The Point Spread Function (PSF) of each fluorophore can be retrieved on both images provided by the two cameras and the measured photon numbers on each camera will be related to the spectral separation of the fluorophore.
Schedule:
10 min – Introduction to Spectral-demixing
15 min – Acquisition of single-color image for control
5 min – Short break to change sample
20min – Simultaneous Multi-color Acquisition using Spectral-demixing
15 min – Summary of the workshop & Questions
ACQUIFER Imaging GmbH
Room: Gallery 1
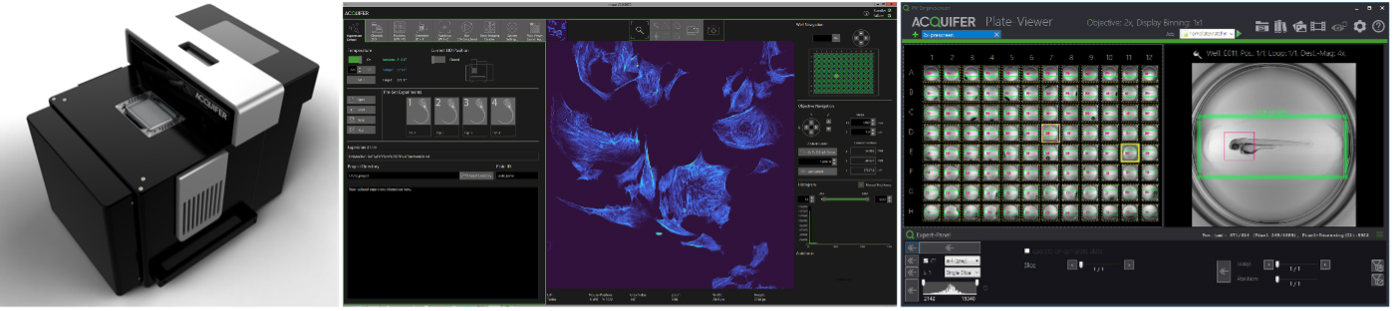
The ACQUIFER Imaging Machine – Automated Imaging, Photomanipulation and High-Content-Screening
Phenotypic screening of complex cellular or whole organism model systems is increasingly employed in biomedical and pharmaceutical research. To fully exploit their potential, novel imaging workflows and tailored tools compatible with the automated imaging and spatiotemporal scoring of cell- and tissue-specific phenotypes are needed.
The ACQUIFER Imaging Machine (IM) is a versatile and flexible automated wide-field fluorescence microscope for high-content imaging of 2D/3D cell-cultures, fixed tissues sections and small model-organisms. The system features a unique moving optical unit in combination with a static sample holder, rendering it ideal for non-adherent and motion-sensitive specimen. For optimal time-lapse in-vivo imaging, the sample chamber is equipped with a Peltier device for highly precise temperature control.
In this workshop, we will demonstrate the unique hardware design and exemplify automated imaging applications. We will illustrate our workflow concept for automated imaging, feedback microscopy and High-Content-Screening using the ACQUIFER IM and associated software tools. Furthermore, we will discuss the optional laser-module that allows scaling-up of challenging photomanipulation experiments, such as photodamaging or photoconversion.
The IM control software ensures simple and intuitive configuration of standard imaging protocols. Complex acquisition procedures can be customized thanks to built-in scripting support (C#-based) or via dedicated extensions for external applications (Fiji, Python etc). The powerful imaging capabilities of the IM are complemented by the ACQUIFER PlateViewer, an easy-to-use software for browsing and visualization of large high-content screening datasets, and an interface for supervised feedback microscopy. The PlateViewer includes a plugin mechanism, allowing interfacing with external software for custom image-analysis. The open interface enables remote-control of the system by external software or integration in a robotic setup. It also allows feedback microscopy workflows for pre-scan/re-scan applications, such as automated centring applications, using Fiji and the PlateViewer.
Refer to our website at www.acquifer.de
ARGOLIGHT
Stand 9
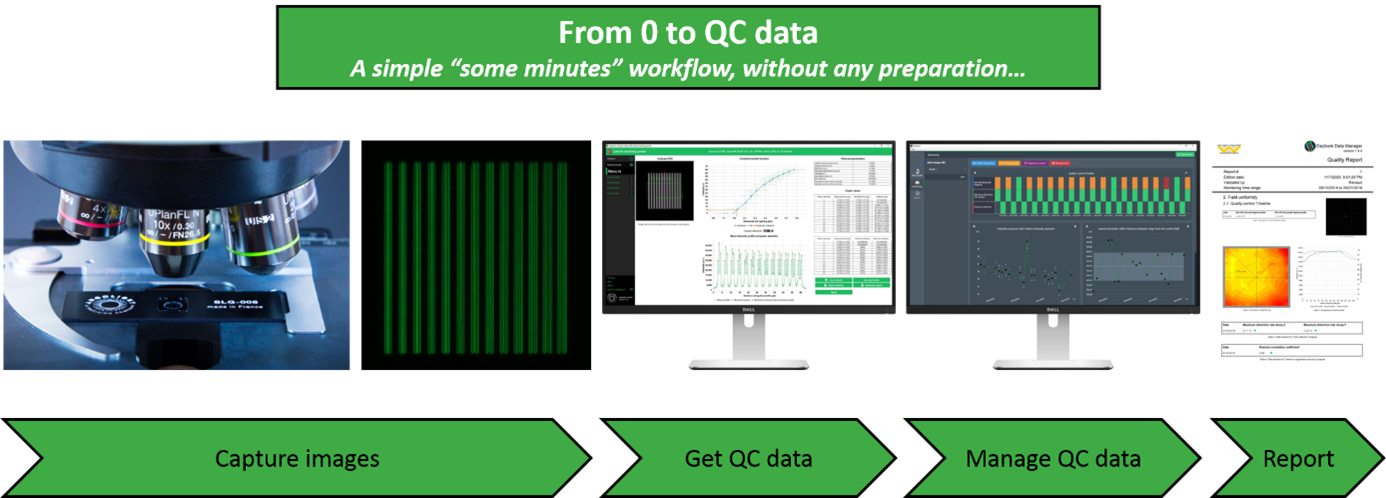
Obtain and manage quality control data on your microscopes
One of the core facilities’ duties is to provide end-users, usually researchers in life sciences, a fleet of microscopes at a level of performance compatible with their experiments. This is not an easy task because the performance of such devices tends to fluctuate or deteriorate over time for many reasons: misusing, aging, environment fluctuations, etc. This is especially true for high end imaging systems such as confocal or super resolution microscopes. Having access to a unique, reliable, and easy-to-use device to ensure microscopes’ performance would certainly make easier this tedious task.
To get quantitative and reproducible data, assessing the performances of fluorescence microscopes is a prerequisite before any imaging campaign. For example, system co-registration accuracy should be evaluated before any co-localization study; System field uniformity and intensity response before any study where intensity in the image matters; Spatial resolution before any study aiming at counting objects close to each other, etc.
Three years after our last presence at ELMI, we are happy to come back and present during this workshop the novelties we have developed in the meantime: improved hardware products, new analyses from 3D patterns, new analyses related to non-Argolight products, improvements in user interface and user experience, etc. Above all, the presentation aims to show how the quality control of fluorescence microscopes can be standardized with Argolight software solutions, and how the generated quality control data can be managed and centralized for later reporting.
Bruker
Room: Goto 32
Imaging across scales – from single particle to whole living and cleared organisms
In this workshop we will demonstrate Bruker super-resolution and light-sheet microscopy solutions (Luxendo SPIM family).
The SPIM demonstration will cover different sample types and potential applications ranging from life imaging of delicate live specimen such as organoids and embryos (mouse, zebrafish etc. ) to whole in-toto imaging of large cleared samples such es entire adult mice. We will be showing our 3D Super-resolution system, the VXL with patented 3D technology suitable for STORM and PALM, along with an integrated
microfluidics system for highly multiplexed, DNA-PAINT research.
Workshop 1/4 : TruLive3D SPIM – light-sheet microscopy for live samples
Workshop 2/5 : VXL – 3D super-resolution microscopy
Workshop 3/6 : LCS SPIM – light-sheet microscopy for cleared samples
Image:
Top, left image: live zebrafish embryo H2A::GFP recorded on Luxendo MuVi SPIM LS.
Top, right image: Mitochondria stained for TOM20 with Alexa 647 and imaged with STORM. Bottom, left image: cleared adult mouse with GFP labeled nerves and recorded on Luxendo LCS SPIM.
Bottom, right: Cleared YFP expressing transgenic mouse brain recorded on Luxendo MuVi SPIM CS
Carl Zeiss Microscopy
Room: Goto 33
Expand your possibilities for High Content Screening from acquisition to data management & analysis
High content screening (HCS) is increasingly adopted for experiments demanding the statistical power of many repeats and the ease of management of thousands of specimens, from acquisition through to automatic analysis, quantification and data management. Whilst drug discovery has been one of the key drivers in the widescale adoption of HCS, many other applications now also benefit from similar approaches for the generation of robust experimental datasets and results.
Robust analysis relies on high image quality of the raw data and powerful image analysis solutions that quickly and reproducibly extract the required data from each image. Celldiscoverer 7 is a powerful HCS boxed microscope solution that uses unique optics to generate unmatched image quality. Teaming these high-quality images with the comprehensive data management solutions provided by arivis VisionHub generates a powerful HCS engine with the flexibility needed for a wide range of applications, from 2D single cells to 3D organoids.
Join this workshop to see the entire HCS workflow in action and experience the benefit of teaming high image quality with powerful data management.
CrestOptics SpA
Stand 34
Improving volumetric deep imaging combining spinning disk and SIM super-resolution microscopy
Fast and highly resolved volumetric microscopy is essential to examine various biological aspects in their complexity, ranging from whole animal imaging, to live phenomena, to thick samples to image at the different depth. During this workshop we demonstrate how X-light V3 spinning disk and DeepSIM add-on can be combined to work with deep samples of thickness comparable to those normally used in CF microscopy. This means that more meaningful data can be obtained from native heterogeneous complex (over 100µm thick) samples using routine preparation protocols.
#deepimaging #clearedtissues #spinningdisk #SIM
Cytena
Stand 8
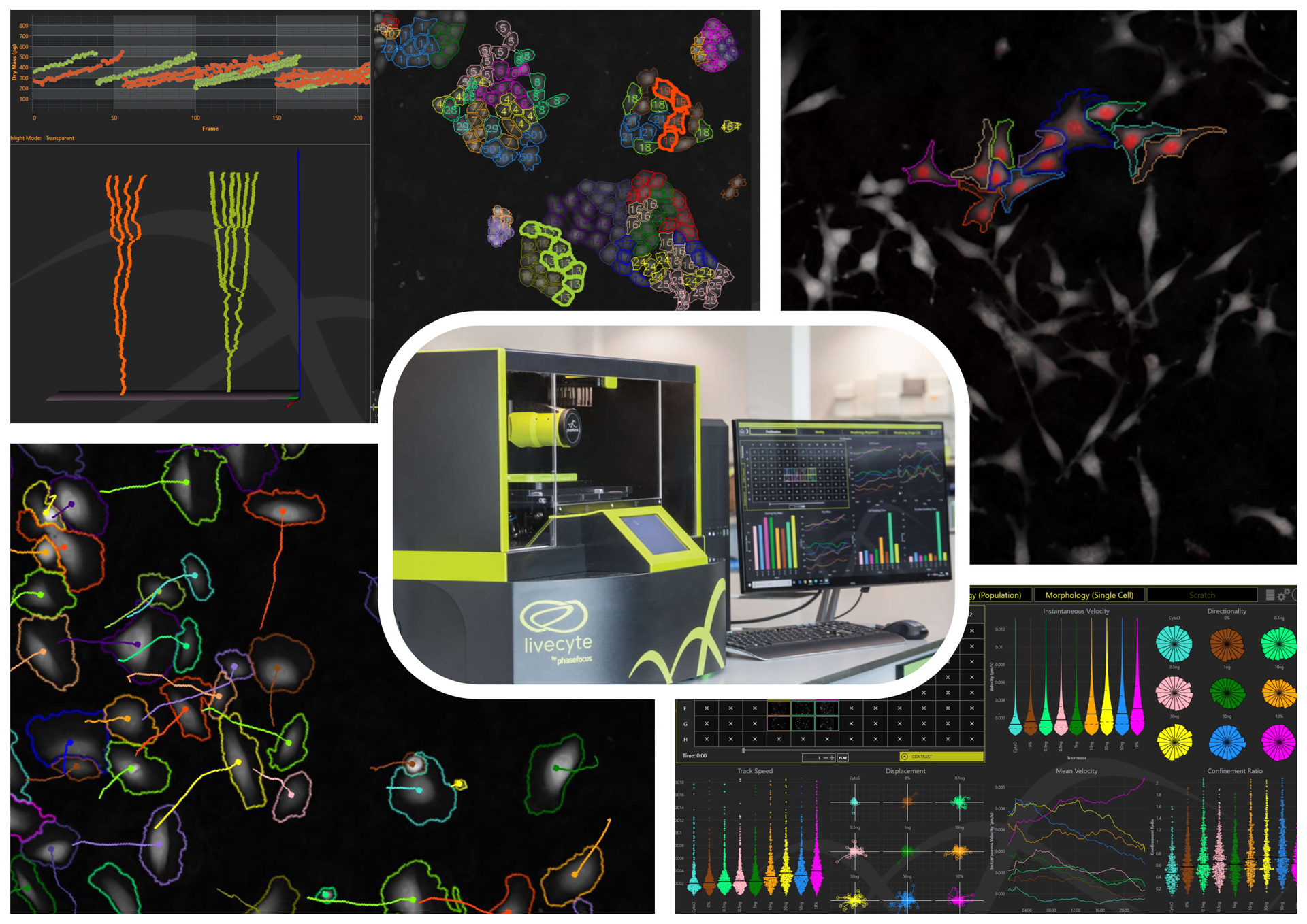
From 96-wells to single cells: Introduction to high content live cell assays on Livecyte
Characterising, comparing, and ultimately understanding, differences in dynamic live cell behaviour is critical in many fields, from cancer research to regenerative medicine.
In this workshop, we explore new ways to characterise cell behaviour, enabled by the Livecyte Kinetic Cytometer system. We look at how simple label-free assays, in 96-well plates, can be used to independently measure cell growth and cell proliferation, automatically characterise both random and sheet cell migration and identify other phenotypic changes. We also show Livecyte’s correlative fluorescence capabilities, and how these measurements unlock additional powerful new insights.
EVIDENT/OLYMPUS
Room: Bäkkäri
Automated Confocal Organoid Imaging
Presenter:
Bülent Peker, Evident Europe
Organoid imaging requires a lot of time, not only capturing images in 3D but also finding target objects especially if the vessel is microplate. With its ability to create excellent low magnifaction images the OLYMPUS confocal laser scanning microscope FLUOVIEW FV3000 has an advantage for organoid imaging, as this feature can be used for creating overview maps to find objects for high resolution imaging with higher magnification.
We automated the process by the integration of an object detection algorithm, and the connection of manual processes into a single step process. This means you can walk away from the system once you set up and start your experiment. No time required to find objects in each well anymore. The FV3000 automatically captures images at low magnification and then finds your objects of interest automatically and captures them at high magnification. This automated process dramatically reduces your time for microscope operation.
ibidi GmbH
Stand 29
ibidi Imaging Chambers and Surfaces
High-resolution microscopy of cells requires imaging chambers that are specifically tailored to your application.
Are you interested in learning about…
… easy to handle all-in-one solutions for live cell imaging?
… the advantages of using channel slides for immunofluorescence stainings?
… solutions for 3D cell culture and spheroid imaging?
Join our workshop and find the answers to these questions!
First, we will introduce a variety of ibidi chambers with different geometries and surfaces and help you to choose the products that are best suited for your assay. This will be followed by a hands-on-training where you get the chance to test a selection of our labware for 2D and 3D cell culture and imaging.
Leica Microsystems CMS GmbH
Room: Teatro
STELLARIS: From Power HyD enhanced photon detection to molecular interactions with TauSense
Confocal microscopy has become a staple for fluorescence imaging in the life sciences, owing to the optical sectioning, sensitivity, and the temporal and spatial resolution capabilities. With STELLARIS, we have introduced key innovations such as the Power HyD family of detectors (1), the next generation of white light lasers (WLL), and the TauSense technology (2).
The fully digital and photon counting proprietary approach, Power Counting, allows straightforward and robust quantification of fluorescence signals in STELLARIS. Together with the WLL excitation flexibility, STELLARIS delivers extended near infrared capabilities for expanding the palette of fluorophores in every experiment., In addition, . TauSense opens the door to functional imaging insights thanks to straightforward access to fluorescence lifetime-based information.
In this workshop we will learn how all the technological innovation in STELLARIS facilitate fluorescence quantifications . We will also unveil a new TauSense tool built to evaluate molecular interactions. Join us for a first-hand view of what STELLARIS can bring to functional imaging.
1. V. Schweikhard, L. A. J. Alvarez, I. Steinmetz, M. J. Roberti, Holger Birk, A. Giske. The Power HyD family of detectors. Nat. Methods. 2020. Doi: d42473-020-00398-0 (2020).
2. M. J. Roberti, L. Ortiz Lopez, G. Ossato, I. Steinmetz, P. Haas, F. Hecht, and L. A. J. Alvarez. TauSense: a fluorescence lifetime-based tool set for everyday imaging. Nat Methods. 2020. Doi: d42473-020-00364-w
Miltenyi Biotec B.V. & Co. KG
Room: Gallery 2
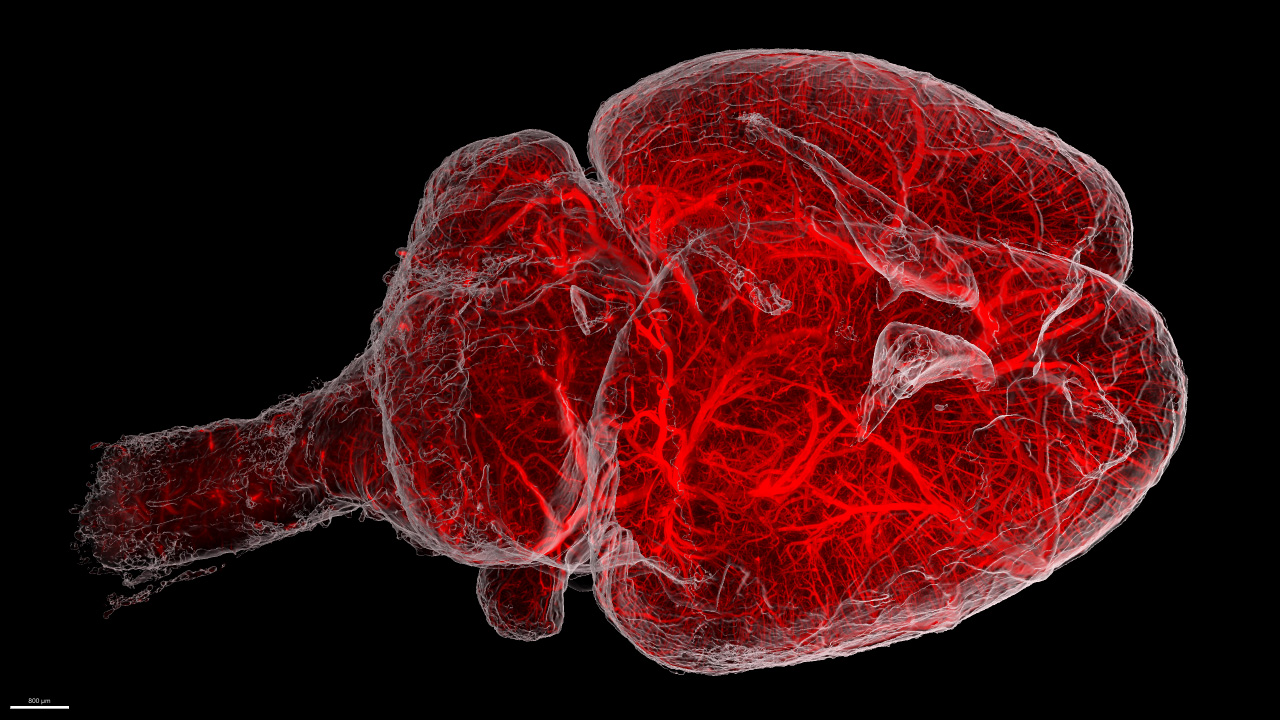
Dive deep into your sample – the Miltenyi 3D imaging workflow
Visualizing the three-dimensional architecture of complex organisms while analyzing biological processes in a whole-organ fashion are becoming the new standard in research. To provide a complete, smooth, and hassle-free 3D imaging workflow, Miltenyi Biotec covers this entire process offering solutions for sample staining, clearing and imaging. In this workshop, we will guide you through the most important steps of sample preparation, including a live demonstration, and will demonstrate how easily high quality 3D images of large samples can be acquired with our cutting-edge light sheet system, the UltraMicroscope BlazeTM.
Nikon Europe BV
Room: Move 1
NIS.ai – Create your own neural network
In this workshop, we will illustrate how deep learning can be implemented in microscopy. Hands-on will be provided with a software environment, which does not require specific computing configuration or programming skills. Examples will include signal to noise and contrast enhancement, image prediction, and segmentation of objects in images. After this workshop, you will be able to train your own neural network with NIS-Elements for your own application. Possible implementation in feedback microscopy will be discussed. One-to-one or small group sessions can be arranged on request. Visit us at our booth and let us know if an additional session works better for your schedule!
Prospective Instruments
Room: Goto 31
First turnkey Multiphoton Microscope!
Bring your own sample to the conference to image it with Prospective Instruments MPX series Two-Photon Microscope. Contact us if you would like to take this opportunity.
Of course, we will also bring a selection of samples to image.
Scientific Volume Imaging
Room: Main Auditorium
Deconvolution, Restoration and Analysis of all your microscopy images Everywhere.
Deconvolution, Restoration and Analysis of all your microscopy images Everywhere.
TissueGnostics GmbH
Room: Logi 1
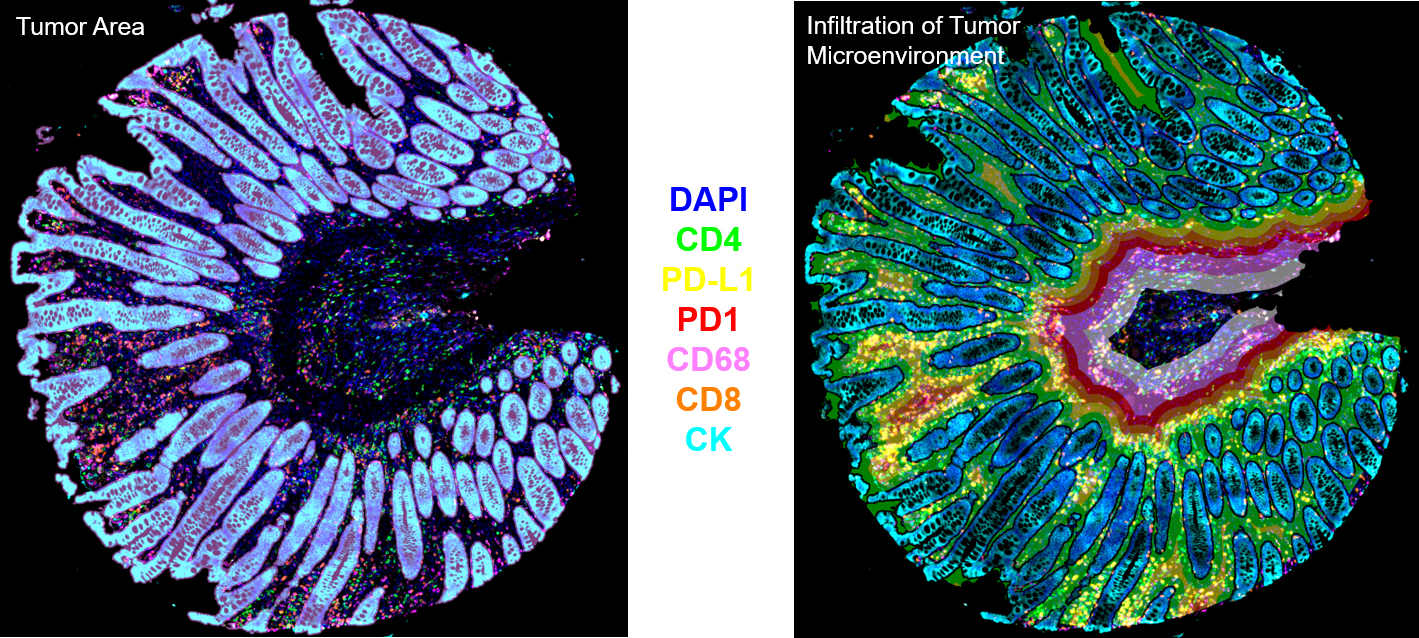
Spatial Tissue Cytometry - Using Precision Microscopy with Artificial Intelligence
Tissue Cytometry permits to determine the in-situ phenotype of cells as well as histological entities, like glands, vessels or tumor foci. Applications include but are not limited to the exploration of the cellular/tumor microenvironment and/or the spatial organization of cellular subpopulations, assessment of different bone structures, quantification of blood vessels and neovascularization as well as analysis of samples in multiplexing or multispectral mode.
Earlier attempts to analyse single cells in tissue have mostly been subject to visual estimation, or – at best – to manual counting for decades. Hence, experts usually had the choice of the “least of evils” between guessing and endless (manual) counting. In (tumor) immunology, infiltrating inflammatory cells need to be phenotypically characterized on a quantitative basis. To better understand the function of inflammatory cells in tumor development, type and number of inflammatory cells and their proximity to glandular/tumor structures have to be analyzed in-situ and correlated with disease state. Using TissueFAXS™ Cytometry the time-consuming and error-prone human evaluation of stained histological sections can be approached with an observer-independent and reproducible technology platform, offering a high degree of automation, paired with user interaction at relevant points of the analytical workflow. TissueFAXS Cytometry is the “one-stop shop” for all kinds of microscopic analysis of tissue sections, organoids and adherent cell cultures.
ONI
Room: Move 2
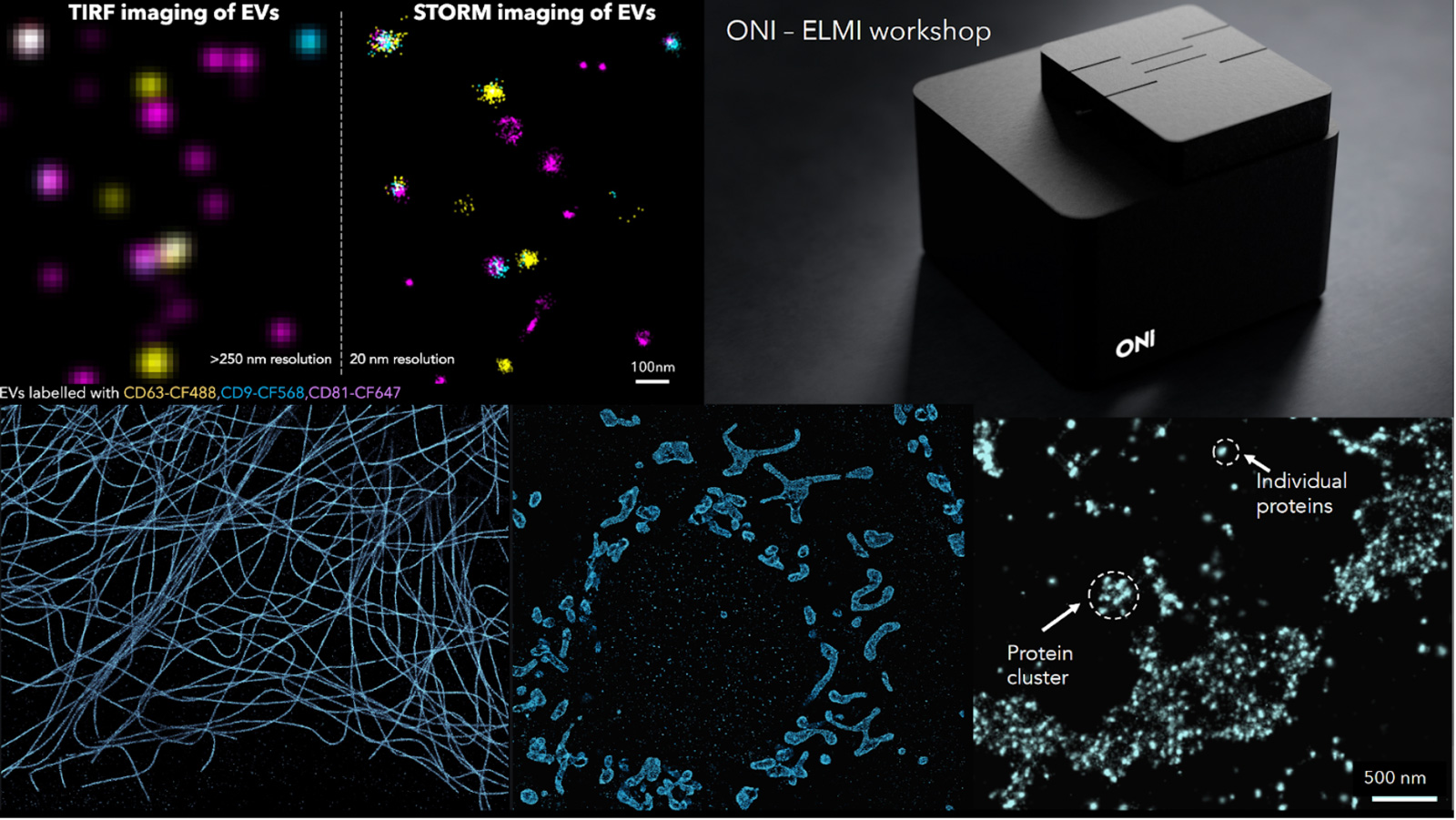
Every Molecule Counts - the ONI super-resolution platform just got even better
Studying spatial protein organization or protein copy number, clustering and mobility at the molecular level is extremely important from both a diagnostic and treatment perspective. Looking at cellular structures beyond the diffraction limit with a resolution of 15-20 nm with robust quantification has been enabled with Single-molecule localization microscopy (SMLM) like STORM or PALM. ONI has created the world’s first desktop super-resolution microscope, the Nanoimager, which is designed to operate on a standard lab bench and has a footprint smaller than a piece of A4 paper, with the most intuitive and simple software interface, making it also accessible to researchers not specialized in high end microscopy techniques with one major goal: make it accessible and simple to use.
It is really important to enable users to extract quantitative information from their localization-based images. We have done this by designing a cloud-based analysis software called CODI (COllaborative DIscovery). CODI helps users analyze their single-molecule data by extracting quantitative information on protein clustering, cluster size and area, cluster density, and so much more. It enables all researchers to upload their data, share or collaborate with other scientists, whilst gaining meaningful insights about their data.
During this workshop we will perform a two-color STORM on mitochondrial samples (TOM20 and HSP60) and reveal the sub-mitochondrial compartmental organization with 20 nm resolution. The aim is to introduce SMLM imaging as a powerful tool for rapid and multiplexed characterization of cellular structures along with a visual and quantification analysis platform CODI developed by us. We will follow up the workshop with an open discussion on the wide-ranging applicability of the Nanoimager in different biological applications encompassing the field of immunology, neurobiology, cell biology and cancer.
Visitron Systems GmbH
Stand 27
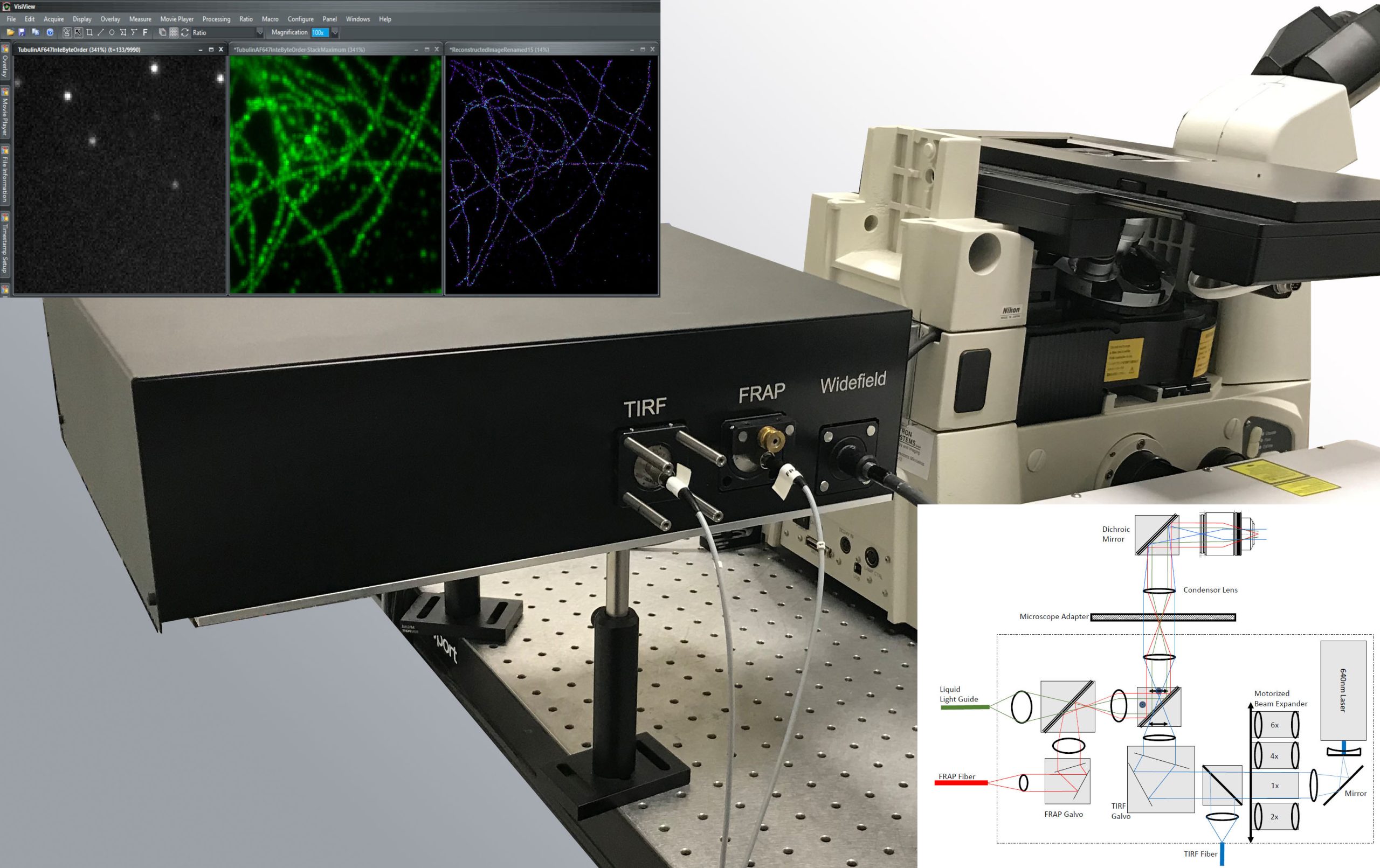
VisiScope 4Elements Flat-Field Confocal Microscopy with FRAP, Ablation and RingTIRFfeaturing a new SoRa-Super Resolution Option
featuring a new SORA-super resolution option
1. AIMS
This workshop will give the listener an overview of new developments in Spinning Disk Microscopy and complementary micromanipulation and imaging methods.
2. SCOPE
Spinning Disk Confocal Microscopy allows researchers to image fast processes in living cells with minimum photobleaching [1]. This makes it an ideal imaging approach to combine with advanced sample manipulation methods like Fluorescence Recovery After Photobleaching (FRAP), Photoactivation (PA) and Ablation.
Over the past two decades, Visitron Systems GmbH has been pioneering new technical developments in this field. One recent result of this effort is an integrated FRAP/Ablation System that can be combined with TIRF and Widefield Imaging all on the same port. At the click of a button, researchers can now cut microscopic structures in 3D space, image membrane processes in TIRF mode and rapidly switch to fast confocal imaging to detect cellular events in confocal mode.
The new Yokogawa-SoRa optics for Super-Resolution based on via Optical Re-Assignment technique with realtime 2D deconvolution processing.