Clearing tissue is now a powerful and accessible technique for studying thicker samples in greater detail. The recent proliferation in tissue-clearing reagents and methods has made the technique more effective and more reliable over a greater range of samples from tissues to organs to whole animals. Once a suitable tissue-clearing regime has been established for a sample the following consideration is what image acquisition modality to use? Two potential options are a spinning disk confocal and a lightsheet microscope, both of which we will present in this workshop. Some of the parameters of a sample or the feature of interest will clearly direct this decision; for example, imaging a whole cleared mouse would typically preclude a spinning disk confocal and imaging a cleared tissue sample at super-resolution would typically preclude lightsheet microscopes. When imaging cleared specimens multiple factors need to be taken into consideration, foremost is a careful evaluation of the volumetric data such that it meets resolution requirements and is an accurate 3D representation of the sample. Other considerations are practical including: safety and ease of sample handling, ease of capture and duration of data acquisition. In this workshop we present our Marianas SDC inverted microscope system featuring a CSU-W SoRa super-resolution spinning disk confocal and our Cleared Tissue LightSheet (CTLS) microscope system and explore cleared tissue samples that could be imaged by each modality and compare the benefits and limitations of each technique.
Workshop 5
THU 9 JUNE 2022, time: 14:30 - 15:30
Click on the images to open the full image view!
Abbelight
Stand 36b
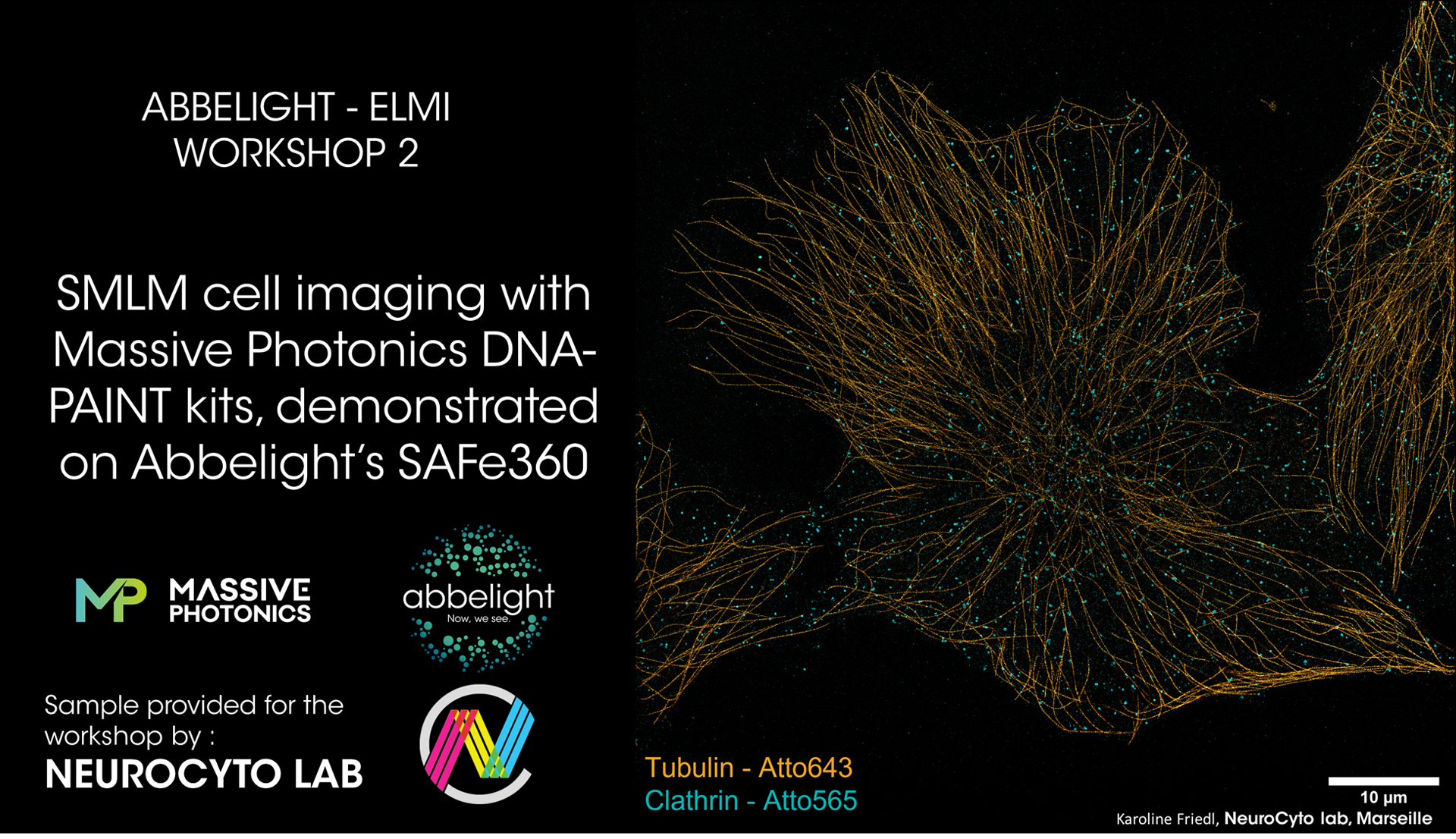
SMLM cell imaging with Massive Photonics DNA-PAINT kits, demonstrated on Abbelight’s SAFe360
Biological imaging has successfully pierced the nanoscale in recent years; resolving the intricate interaction of multiple targets in a system naturally represents the next step in the evolution of fluorescence nanoscopy. For Single molecule localization microscopy (SMLM) , one of the most promising ideashas been to spectrally demix the fluorescence emission of several dyes with similar spectral properties, mostly applied toStochastic optical reconstruction microscopy (STORM). Meanwhile,DNA-PAINT (DNA-Point accumulation for imaging in nanoscale topography) inherentlyholds great potential for multicolor imaging due to its different approach to attaining the “blinking” required for SMLM. DNA-PAINT relies on the rapid hybridization and dehybridization of short complementary DNA strands, retaining a dye long enough close to the target to collect a few thousand photons and “localize it” before the dye diffuses off again. DNA-PAINT imagers (dye coupled to the DNA strand) are independent of the secondary antibody which enables imaging with reduced photobleaching, flexible adjusting of the imager’s concentration or replacing it with another imager With the specificity of the DNA imager strands, nearly limitless multiplexing of different targets with just a few laser sources is possible. Massive Photonics provides DNA-PAINT staining kits consisting of secondary antibodies or single-domain antibodies and complementary imagers, easy to integrate into a regular immuno-labeling routine.
With this workshop, we would like to demonstrate the versatility in multiplexing that the combination of Massive Photonics staining kits with the Abbelight SAFe360 setup yields: From consecutive imaging of multiple targets, over simultaneous two-color imaging to spectral demixing. We will show the imaging procedure of an exemplary one and/or two-color imaging on Cos7 cells or cultured hippocampal rat neurons
Schedule:
15 min – Introduction: Massive Photonics kits,samples andsetup (Presentation)
15 min – Acquisition on a one-color image, explanation of the imaging parameters
5 min – Short break to change or add an imager
15 min – Acquisition of the second color or two colors at once
10 min- Summary of the workshop & Questions
ACQUIFER Imaging GmbH
Room: Gallery 1
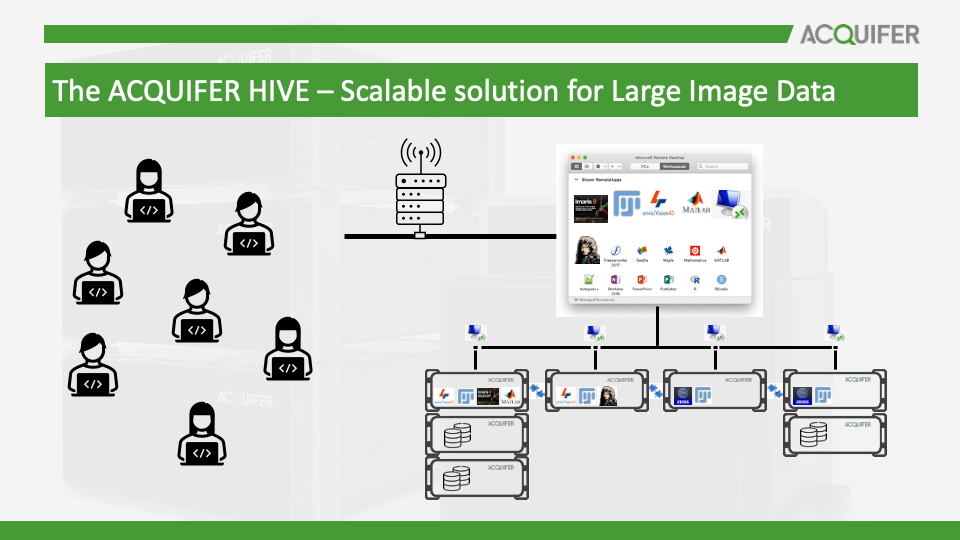
The ACQUIFER HIVE - Solution for Large Image Data
Modern microscopy techniques acquire an ever-increasing amount of multidimensional image data.
3D microscopy techniques, such as selective plane illumination microscopy (SPIM), easily generate multiple terabytes of raw image data in a single experiment that require tailored solutions for interactive or automated analysis.
The ACQUIFER HIVE is the established solution for efficient and safe management and storage of large image data without USB drives or network copy. The HIVE allows fast access to acquired data for swift processing and flexible analysis workflows. It is designed as a modular platform that is configured for your specific applications and will grow with your needs.
In this workshop, we will introduce the concepts of HIVE workflows, and we will present new solutions to expand existing HIVE installations to serve a higher user base and larger projects. Multi-GPU and Multi-HIVE solutions are outlined. Besides scaling in processing, concepts about data safety with large storage pools are discussed.
As part of the workshop, we offer RDP access on the centralized multi user system HIVE for testing. We encourage participants to bring their own laptops.
Please refer to our website at www.acquifer.de or call us at +49 (6221) 435 2000 for more detailed information on ACQUIFER products.
ARGOLIGHT
Stand 9
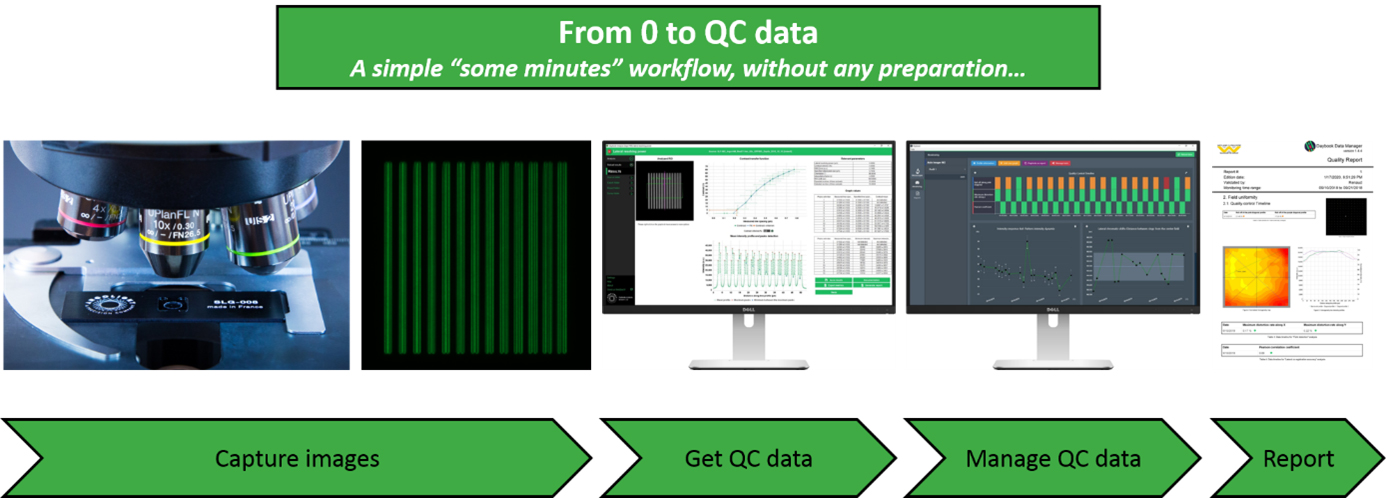
Obtain and manage quality control data on your microscopes
One of the core facilities’ duties is to provide end-users, usually researchers in life sciences, a fleet of microscopes at a level of performance compatible with their experiments. This is not an easy task because the performance of such devices tends to fluctuate or deteriorate over time for many reasons: misusing, aging, environment fluctuations, etc. This is especially true for high end imaging systems such as confocal or super resolution microscopes. Having access to a unique, reliable, and easy-to-use device to ensure microscopes’ performance would certainly make easier this tedious task.
To get quantitative and reproducible data, assessing the performances of fluorescence microscopes is a prerequisite before any imaging campaign. For example, system co-registration accuracy should be evaluated before any co-localization study; System field uniformity and intensity response before any study where intensity in the image matters; Spatial resolution before any study aiming at counting objects close to each other, etc.
Three years after our last presence at ELMI, we are happy to come back and present during this workshop the novelties we have developed in the meantime: improved hardware products, new analyses from 3D patterns, new analyses related to non-Argolight products, improvements in user interface and user experience, etc. Above all, the presentation aims to show how the quality control of fluorescence microscopes can be standardized with Argolight software solutions, and how the generated quality control data can be managed and centralized for later reporting.
Bruker
Room: Goto 32
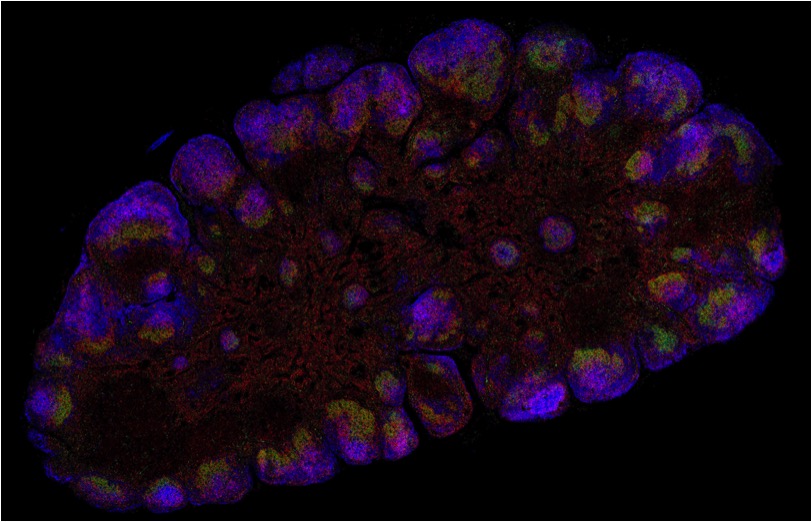
Imaging across scales – from single particle to whole living and cleared organisms
In this workshop we will demonstrate Bruker super-resolution and light-sheet microscopy solutions (Luxendo SPIM family).
The SPIM demonstration will cover different sample types and potential applications ranging from life imaging of delicate live specimen such as organoids and embryos (mouse, zebrafish etc. ) to whole in-toto imaging of large cleared samples such es entire adult mice. We will be showing our 3D Super-resolution system, the VXL with patented 3D technology suitable for STORM and PALM, along with an integrated
microfluidics system for highly multiplexed, DNA-PAINT research.
Workshop 1/4 : TruLive3D SPIM – light-sheet microscopy for live samples
Workshop 2/5 : VXL – 3D super-resolution microscopy
Workshop 3/6 : LCS SPIM – light-sheet microscopy for cleared samples
Image:
Top, left image: live zebrafish embryo H2A::GFP recorded on Luxendo MuVi SPIM LS. Top, right image: Mitochondria stained for TOM20 with Alexa 647 and imaged with STORM. Bottom, left image: cleared adult mouse with GFP labeled nerves and recorded on Luxendo LCS SPIM. Bottom, right: Cleared YFP expressing transgenic mouse brain recorded on Luxendo MuVi SPIM CS
Carl Zeiss Microscopy
Room: Goto 33
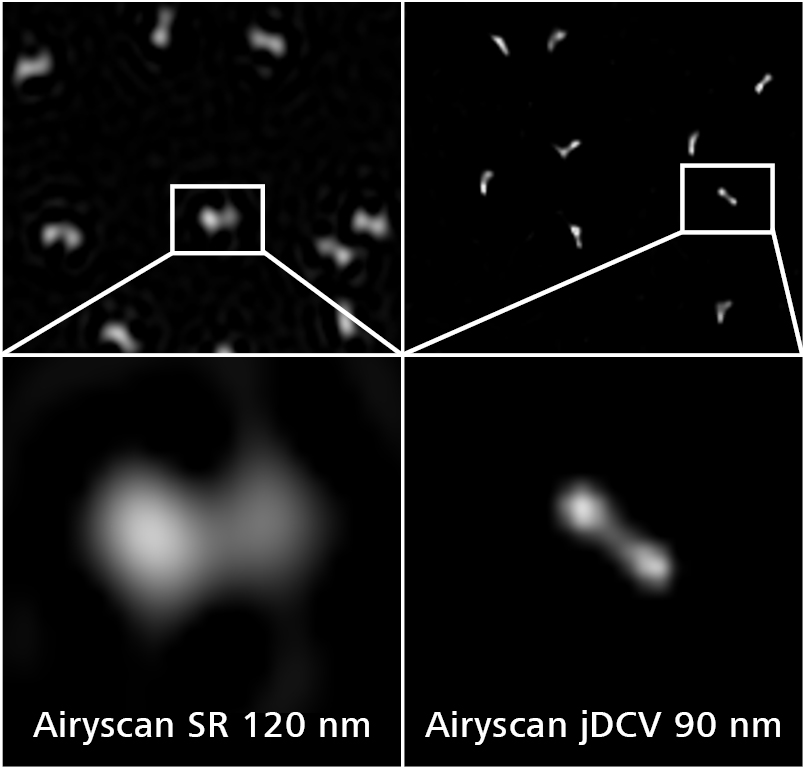
Enjoy better confocal performance in every imaging mode
Incremental improvements in confocal imaging have assisted the flexibility of the laser scanning microscope, but these are often limited to a single acquisition mode.
Two new capabilities for the ZEISS confocal microscopes provide improvements in image quality for every imaging modality. LSM Plus works with all detectors to both improve signal to noise ratio and increase image resolution down to 120nm and Airyscan Joint Deconvolution (jDCV) uses the Airyscan technology to generate image resolutions down to 90nm. Both capabilities provide these significant image improvements without the need for additional detectors or imaging approaches. These developments boost the performance to such an extent that your confocal now provides an easy path to super resolution without needing to move to alternative imaging platforms.
This workshop will introduce the new functionalities and explore the details of how these capabilities can be easily used to gain additional insights from a wide range of specimens.
CrestOptics SpA
Stand 34
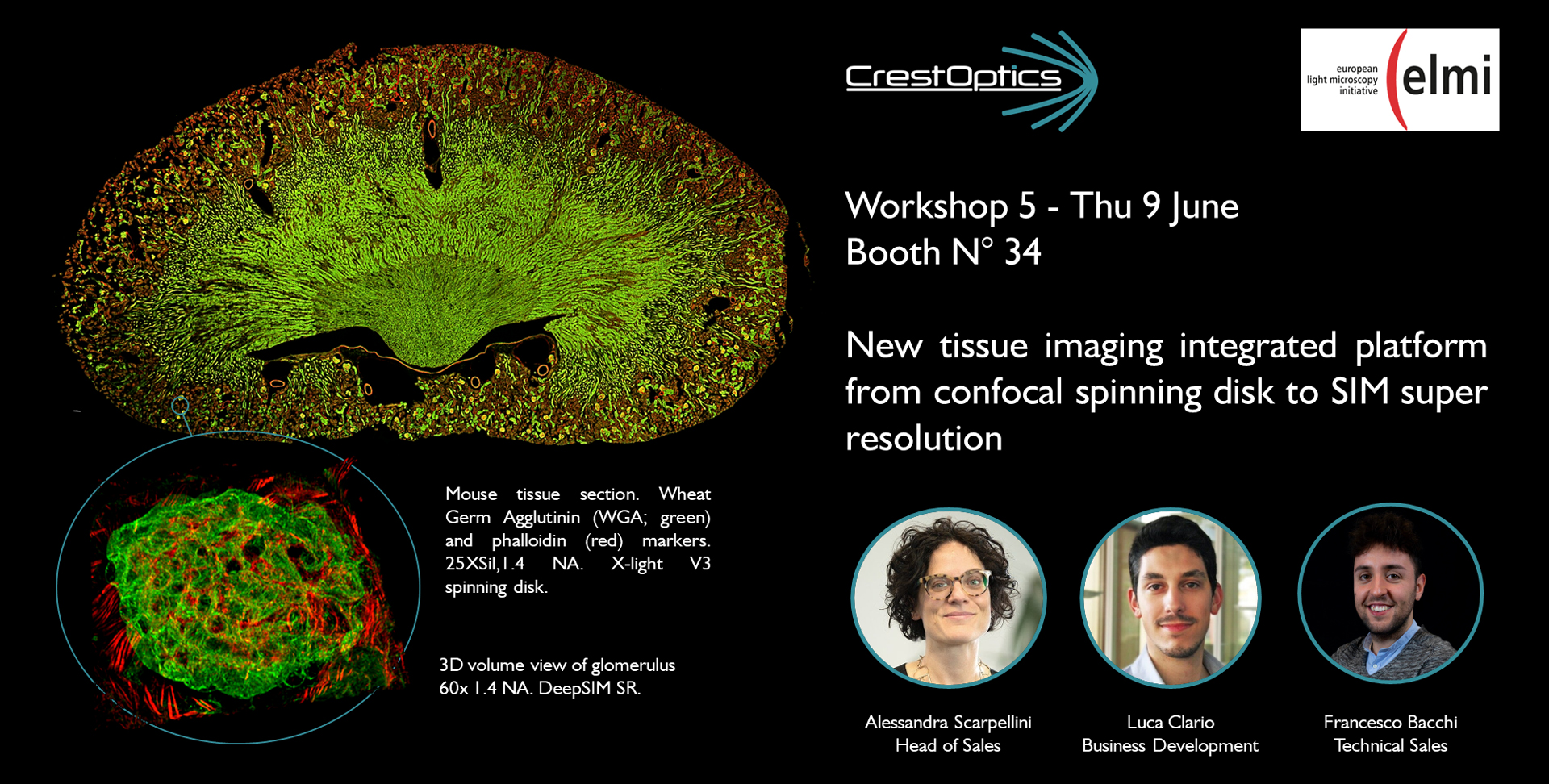
New tissue imaging integrated platform from confocal spinning disk to SIM super resolution
X-Light V3 spinning disk provides highly homogeneous illumination over the entire 25 mm field of view on two cameras simultaneously, it gives the opportunity for seamless stitching of images of very large tissues allowing to get reliable data without vignetting and increasing speed by minimizing tiles overlap. Thanks to the new DeepSIM add-on, affordable easy-to-use low magnification dry objectives can also be used to acquire both conventional and not-conventional specimens, doubling the objective performance and obtaining an image quality very similar to that reached with confocal imaging with oil immersion lenses. In this workshop we demonstrate that this integrated solution greatly increases the flexibility of any imaging system and provides a powerful platform for high-throughput confocal highly resolved tissue imaging.
#tissueimaging #spinningdisk #SIM #seamless_stiching
Cytena
Stand 8
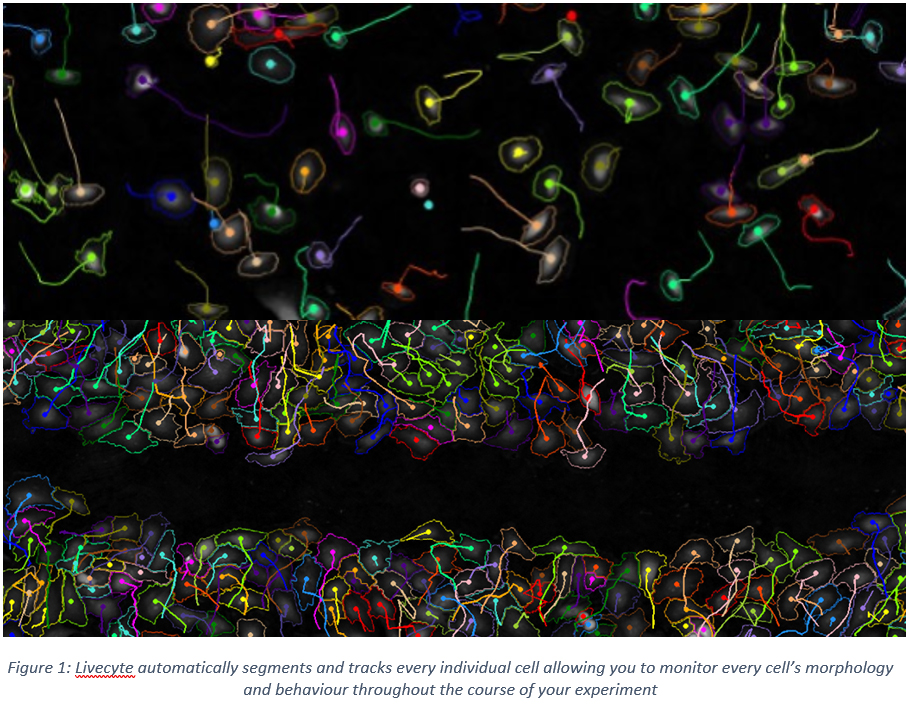
Measuring Random Motility and Wound Healing with Livecyte: are you only scratching the surface?
Dreading another day of monotonous mouse clicking to manually track cells? What if your derived motility measurements are not as reliable as you think? Livecyte can automatically track thousands of cells presenting clear advantages in the accuracy of motility metrics, as well as saving a considerable amount of time and energy.
In this workshop we will explore some of the sources of error when manually tracking live cells, in particular the variability from user to user and the lack of consistency and accuracy in clicking on the centre of a cell. These errors lead to discrepancies in derived cell track lengths. We reveal how Livecyte overcomes these limitations with its standardised automated tracking algorithm shown to alleviate the high level of variation between users manually tracking the same cells. In addition, by accurately tracking and analysing individual cells, Livecyte can give a greater depth of information such as speed and directionality. All these metrics and data are conveniently organised into Livecyte’s Motility and Wound Healing Dashboards which make it easy for users to understand the motility behaviour of their cells.
EVIDENT/OLYMPUS
Room: Bäkkäri
Automated Confocal Organoid Imaging
Presenter: Bülent Peker, Evident Europe
Organoid imaging requires a lot of time, not only capturing images in 3D but also finding target objects especially if the vessel is microplate. With its ability to create excellent low magnifaction images the OLYMPUS confocal laser scanning microscope FLUOVIEW FV3000 has an advantage for organoid imaging, as this feature can be used for creating overview maps to find objects for high resolution imaging with higher magnification.
We automated the process by the integration of an object detection algorithm, and the connection of manual processes into a single step process. This means you can walk away from the system once you set up and start your experiment. No time required to find objects in each well anymore. The FV3000 automatically captures images at low magnification and then finds your objects of interest automatically and captures them at high magnification. This automated process dramatically reduces your time for microscope operation.
ibidi GmbH
Stand 29
ibidi Imaging Chambers and Surfaces
High-resolution microscopy of cells requires imaging chambers that are specifically tailored to your application.
Are you interested in learning about…
• … easy to handle all-in-one solutions for live cell imaging?
• … the advantages of using channel slides for immunofluorescence stainings?
• … solutions for 3D cell culture and spheroid imaging?
Join our workshop and find the answers to these questions!
First, we will introduce a variety of ibidi chambers with different geometries and surfaces and help you to choose the products that are best suited for your assay. This will be followed by a hands-on-training where you get the chance to test a selection of our labware for 2D and 3D cell culture and imaging.
Leica Microsystems CMS GmbH
Room: Teatro
Enter the Microhub Era - Meet Mica!
Experience Mica in action and learn how its sample-centric operation allows straightforward access to imaging for all users .
See how MICA dramatically reduces the time-to-image and training time for non-imaging specialists.
Learn how our new FluoSync technology detects four fluorophores simultaneously, in both widefield and confocal!
Discover how the whole workflow from setting up an imaging experiment to the AI powered extraction of data has been radically simplified.
See all this working directly on samples and specimens you see every day – from multi-well plate assays to large tissue sections to model organisms – all on Mica!
Cutrale, F., Trivedi, V., Trinh, L. et al. Hyperspectral phasor analysis enables multiplexed 5D in vivo imaging. Nat Methods 14, 149–152 (2017). https://doi.org/10.1038/nmeth.4134
·Hsiao Chiang, Daniel Koo, Masahiro Kitano et al. HyU: Hybrid Unmixing for longitudinal in vivo imaging of low signal to noise fluorescence, 12 January 2022, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-1073331/v1]
White paper: https://webcdn.leica-microsystems.com/fileadmin/global/products/Supercharge-the-way-you-work/White_paper_FluoSync_MC-0003306.pdf
Nikon Europe BV
Room: Move 1
Expand to lifetime imaging: A seamless integration of AX point scanner confocal and FLIM
With AX’s new capabilities and easily managed lifetime experiment combination, we add an extra dimension to confocal microscopy.
In this workshop, you will find out how one can acquire and visualize FLIM data with the help of NIS-Elements. Discover a quick and intuitive basic analysis package directly included in NIS-Elements: Large image, Z stack, automated complex acquisitions, and basic analysis. Easy export of FLIM image to Symphotime within one click for more in-depth is at reach.
There is much more to discover, so join us and learn how AX/FLIM provides seamlessly integrated lifetime acquisition with unprecedented resolution.
Prospective Instruments
Room: Goto 31
First turnkey Multiphoton Microscope!
Bring your own sample to the conference to image it with Prospective Instruments MPX series Two-Photon Microscope. Contact us if you would like to take this opportunity.
Of course, we will also bring a selection of samples to image.
Rapp OptoElectronic GmbH
Stand 35
FLUCS: Micro flow photomanipulation
FLUCS photomanipulation is a non-invasive, all-optical method based on thermoviscous flow that allows the microscopist to induce precise microscopic flow fields inside living cells or microfluidic chambers.
During the workshop, we present RAPP’s FLUCS add-on optical module that is compatible with all standard inverted microscopes,we explain the working principle of FLUCS technology, we discuss a variety of recent applications of this novel technology in the life sciences, and we provide guidance, to optimally exploit the technology for your experiment.
www.rapp-opto.com/products/photomanipulation-systems/flucs-micro-flow-photomanipulation/
Thermo Fisher Scientific
Room: Goto 30
Explore large multi-channel and time series data with Amira Software and the new Xplore5D extension.
During these workshops you will discover, through a step-by-step demo, the recently added functions of the Thermo Scientific™ Amira™ Software’s new Xplore5D extension, which offers data conversion and compression with immediate visual feedback, allowing you to quickly interact with and communicate on large 3–5D data.
TissueGnostics GmbH
Room: Logi 1
Exploring the World of Multiplexing Immunofluorescence in Human Immune System Studies
Distinguished Guest Speaker Thomas Diefenbach, The Ragon Institute Boston
In fields such as immunology, where methods such as flow cytometry typically employ 15 to 30 markers per cell, the necessity for characterizing cell populations similarly in tissue is limited by the number of markers one can interrogate using conventional epifluorescence or confocal microscopy approaches. In this virtual workshop, various approaches for multiplexing fluorescent markers in tissue will be combined with the TissueFAXS high-throughput automated slide scanning systems by TissueGnostics to demonstrate the ease with which one may examine far more than the standard four or five fluorophores at the single cell level. Potential applications for such approaches will also be outlined that examine cell-to-cell interactions and dynamics in tissues in a highly regional and quantitative manner utilizing analysis tools afforded by the TissueQuest and StrataQuest analysis software packages.
University of Dundee/OME
Room: Main Auditorium
Achieving the FAIR vision in imaging
Have you ever wanted to find existing data to reuse? This workshop explores the workflow where a need for images as a resource for reanalysis, training of users, training of algorithms and quality control is addressed by using the Image Data Resource (IDR), whose main purpose is making the data FAIR. First, an example workflow from looking for data in IDR, using these data in analysis workflows, exploring the possibilities of downloading images and metadata from IDR and storing the results will be shown. Further, the way forward in this workflow using the ome-zarr will be shown in examples and the need for software adaptations to accommodate ome-zarr discussed.
ONI
Room: Move 2
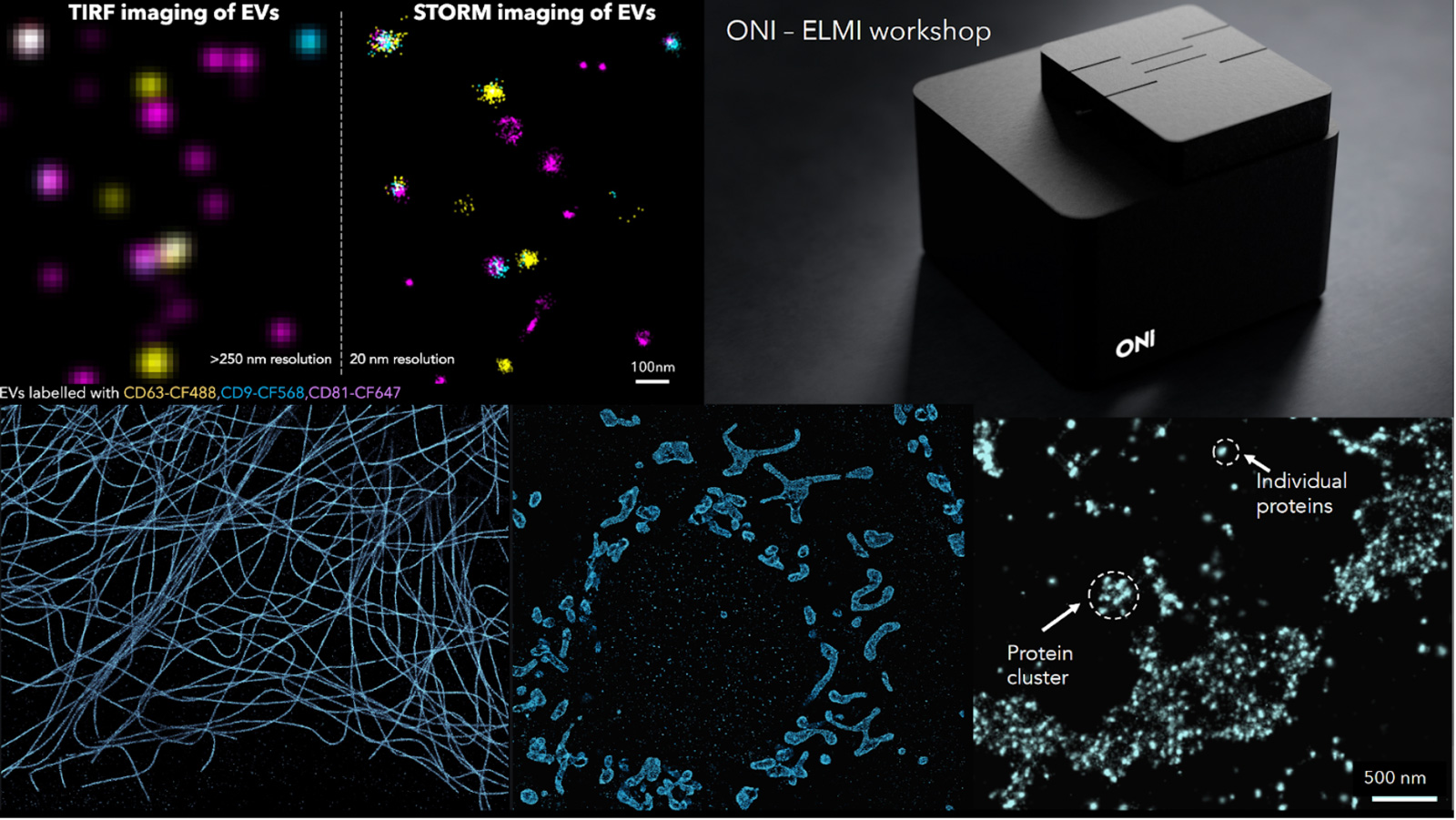
Every Molecule Counts - the ONI super-resolution platform just got even better
Studying spatial protein organization or protein copy number, clustering and mobility at the molecular level is extremely important from both a diagnostic and treatment perspective. Looking at cellular structures beyond the diffraction limit with a resolution of 15-20 nm with robust quantification has been enabled with Single-molecule localization microscopy (SMLM) like STORM or PALM. ONI has created the world’s first desktop super-resolution microscope, the Nanoimager, which is designed to operate on a standard lab bench and has a footprint smaller than a piece of A4 paper, with the most intuitive and simple software interface, making it also accessible to researchers not specialized in high end microscopy techniques with one major goal: make it accessible and simple to use.
It is really important to enable users to extract quantitative information from their localization-based images. We have done this by designing a cloud-based analysis software called CODI (COllaborative DIscovery). CODI helps users analyze their single-molecule data by extracting quantitative information on protein clustering, cluster size and area, cluster density, and so much more. It enables all researchers to upload their data, share or collaborate with other scientists, whilst gaining meaningful insights about their data.
During this workshop we will perform a two-color STORM on mitochondrial samples (TOM20 and HSP60) and reveal the sub-mitochondrial compartmental organization with 20 nm resolution. The aim is to introduce SMLM imaging as a powerful tool for rapid and multiplexed characterization of cellular structures along with a visual and quantification analysis platform CODI developed by us. We will follow up the workshop with an open discussion on the wide-ranging applicability of the Nanoimager in different biological applications encompassing the field of immunology, neurobiology, cell biology and cancer.
Visitron Systems GmbH
Stand 27
VisiScope 4Elements Flat-Field Confocal Microscopy with FRAP, Ablation and RingTIRF featuring a new SoRa-Super Resolution Option
1. AIMS
This workshop will give the listener an overview of new developments in Spinning Disk Microscopy and complementary micromanipulation and imaging methods.
2. SCOPE
Spinning Disk Confocal Microscopy allows researchers to image fast processes in living cells with minimum photobleaching [1]. This makes it an ideal imaging approach to combine with advanced sample manipulation methods like Fluorescence Recovery After Photobleaching (FRAP), Photoactivation (PA) and Ablation.
Over the past two decades, Visitron Systems GmbH has been pioneering new technical developments in this field. One recent result of this effort is an integrated FRAP/Ablation System that can be combined with TIRF and Widefield Imaging all on the same port. At the click of a button, researchers can now cut microscopic structures in 3D space, image membrane processes in TIRF mode and rapidly switch to fast confocal imaging to detect cellular events in confocal mode.
The new Yokogawa-SoRa optics for Super-Resolution based on via Optical Re-Assignment technique with realtime 2D deconvolution processing.